Israeli Study Reports 100% One-Year Survival Using New Protocol for Advanced Hodgkin’s
‘A true revolution in Hodgkin lymphoma care.’
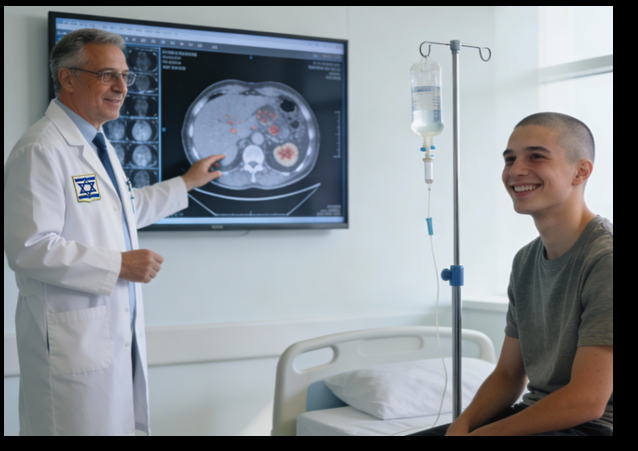
Israeli researchers have reported a new treatment for advanced‑stage classical Hodgkin lymphoma (a type of cancer of the white blood cells) that achieved a 100% one‑year overall survival rate in a large cohort of patients participating in their program.
Israeli researchers have unveiled an innovative treatment for advanced-stage Hodgkin lymphoma that they say resulted in a one-year survival rate of 100 per cent, representing a “true revolution” in caring for people with the disease.
While traditionally the management of patients with advanced-stage classical Hodgkin lymphoma has relied exclusively on chemotherapy, the new treatment combines advanced chemotherapy with targeted biological therapy.
The groundbreaking study was conducted across 15 Israeli medical centres. Of 79 patients, 95 percent of experienced complete response to the treatment – meaning all detectable signs of a disease disappeared, with just four per cent of participants requiring additional radiation therapy, according to the results, published in the medical journal, Blood.
The treatment also appeared to cause fewer, and less harsh, side effects compared with traditional approaches.
BREAKING: Israeli scientists just delivered a miracle at the world’s top cancer conference, a new treatment for Hodgkin lymphoma showing a 100% survival rate.
✅ Almost no radiation needed
✅ Real results from 15 hospitals across Israel
✅ Signs of cancer gone after just 9 weeks… pic.twitter.com/NmKGO3Wrdy— Hananya Naftali (@HananyaNaftali) December 10, 2025
The protocol is a combined “chemo‑biologic regimen” (often referred to as the BREcADD protocol) that was based on a prior German trial that was then further modified by the Israeli researchers.
Dr. Roy Vitkon of Ichilov Medical Center said expectations for the protocol rose sharply after a major German study published in The Lancet last year showed near-universal cure rates. He noted that earlier regimens were effective but came with harsh side effects, while the new approach appeared both stronger and easier for patients to tolerate. Vitkon said Israel moved quickly to test the method in routine clinical practice, gathering data from nearly 100 patients over the past two years.
According to Vitkon, Israel was the first country to produce real-world data confirming the protocol’s effectiveness. He described the multi-center cooperation as an achievement in its own right, saying the strong alignment with the German study gives physicians confidence to continue adopting the treatment.
In a multi-center Israeli trial for advanced-stage Hodgkin lymphoma, 95% of patients had a complete response and the 1-year survival rate was 100%. Chemo plus targeted biological therapy. While the world screams about Israel, its doctors quietly rewrite cancer care.…
— Trisha Posner (@trishaposner) December 12, 2025
Hodgkin lymphoma can occur in both children and adults, but it appears most often in young adults, particularly those in their 20s. The likelihood of developing Hodgkin lymphoma increases again later in life, starting around age 55.
The American Cancer Society’s estimates for Hodgkin lymphoma in the United States for 2025 are:
- About 8,720 new cases (4,840 in males and 3,880 in females)
- About 1,150 deaths (720 males and 430 females)
The initial signs of this disease include swollen lymph nodes that are painless at first. Ongoing systemic effects can include increasing fatigue, loss of appetite, more pronounced weight loss, persistent fevers and night sweats, and sometimes itching, bone pain, or symptoms from bone marrow involvement (e.g., frequent infections, easy bruising, or worsening anemia).
Interestingly, last year, there was a report about a successful new treatment strategy involving the immunotherapy drug nivolumab along with three different chemotherapies. The protocol was found to result in a two-year survival rate of 92% in a phase three trial whose results were published in the New England Journal of Medicine.
Like the Israeli study, the test treatments were coordinated at numerous medical sites.
The new study included nearly 1,000 people who were at least 12 years old and newly diagnosed with stage III or IV Hodgkin lymphoma that had previously been untreated.
Between July 2019 and October 2022, the patients were randomly assigned to receive one of two treatment regimens; 496 patients were assigned to receive the antibody drug brentuximab vedotin along with the chemotherapy drugs doxorubicin, vinblastine and dacarbazine, while 498 others received nivolumab along with that same trio of chemotherapies.
The patients received the treatments intravenously on the first day and then about two weeks later within a 28-day cycle. This was repeated for six cycles.
The patients were based at 256 sites across the United States and Canada. One of the sponsors of the trial was Bristol-Myers Squibb, which makes nivolumab and supplied the drug for the study.
It is heartening to know that modern medicine can still create seemingly miraculous levels of healing!
Image by perplexity.ai
 DONATE
DONATE
Donations tax deductible
to the full extent allowed by law.









Comments
I presume all BDS antisemite will forego this treatment.
hey will call it “voodoo medicine”. Biologics are already in use. This uses a different mix and builds on history of R-CHOP. How far we’ve come from the 100% death sentence.
Just like they fireho Jewish attorneys when they really REALLY need a good mouthpiece.
How speech to text turned “hire” into “fireho” is a mystery for the ages.
Many “Palestinian’s” went to Israel hospitals for treatment prior to 10/7
Israel/Jews advance science and medicine. Leftists and Muslims destroy and would destroy Israel and kill all the Jews if they could. Gee. Who should I side with? Bueller? Bueller?
I don’t know the number, but I’d be willing to bet that some of the patients treated in the Israeli study were other than the standard Israeli Jew; some might even have been Israeli/”Palestinian” Muslims.
Some of the doctors were likely muslim
Candace Owens makes commentary in 3 / 1…
Followed by TC?
Followed by NF?
That’s cool. Dont share it with any of the countries not participating in Eurovision next year 😂😂
Can someone tell me – how many muslims have advanced medicine or science in the last 600-700 years?
Aside from all the good of this….
Why does the doctor look like Fauci?
Leave a Comment